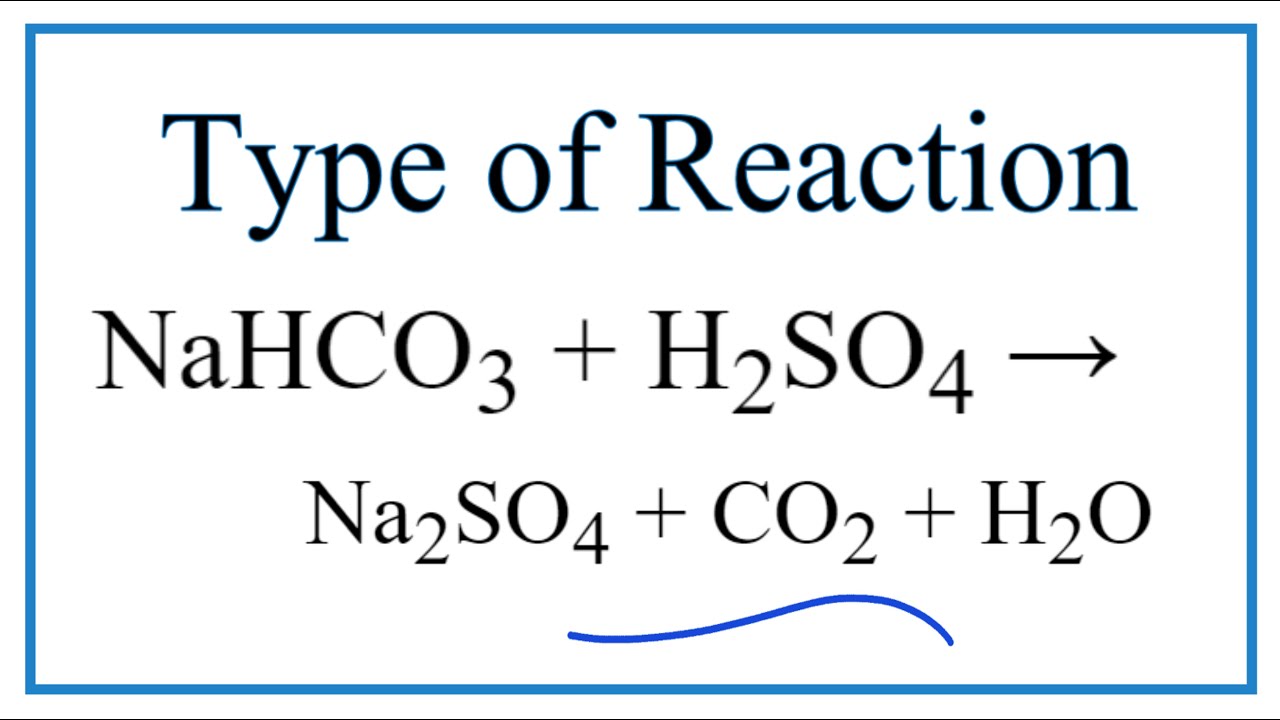
Write the balanced ionic equation for the reaction of sodium bicarbonate with sulphuric acid - YouTube

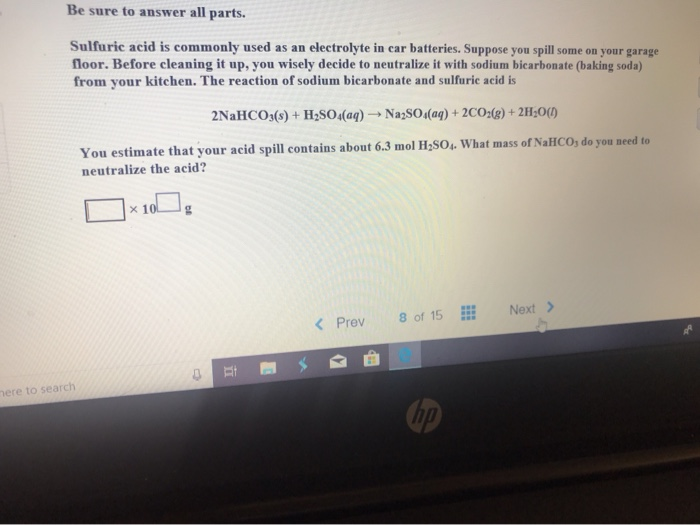
SOLVED: Be sure t0 answer all parts Sulfuric acid is commonly used as an electrolyte in car batteries. Suppose you spill some on your garage floor: Bcfore cleaning it UP, YOU wisely

SOLVED: Some sulfuric acid is spilled on a lab bench. You can neutralize the acid by sprinkling sodium bicarbonate on it and then mopping up the resultant solution. The sodium bicarbonate reacts
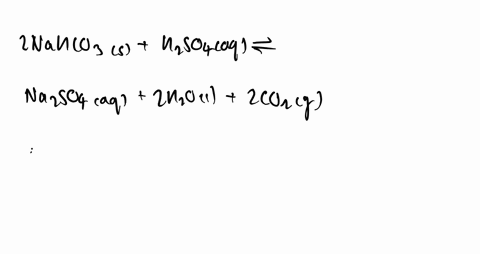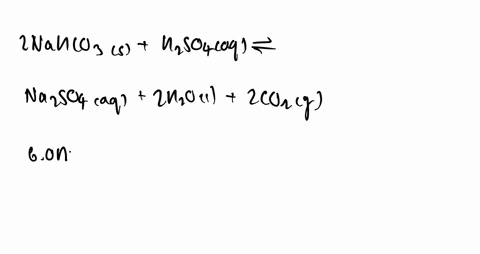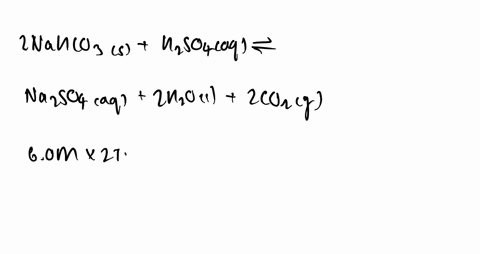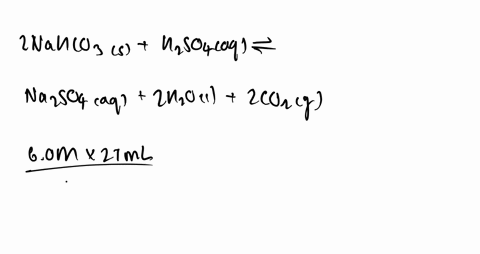
SOLVED:Some sulfuric acid is spilled on a lab bench. You can neutralize the acid by sprinkling sodium bicarbonate on it and then mopping up the resultant solution. The sodium bicarbonate reacts with